List the Four Possible Subshells in the Quantum Mechanical Model
The quantum numbers of an electron are kind of like the electrons address. 2 6 10 14.

Energy Levels And Orbitals Ppt Video Online Download
Need a deep-dive on the concept behind this application.
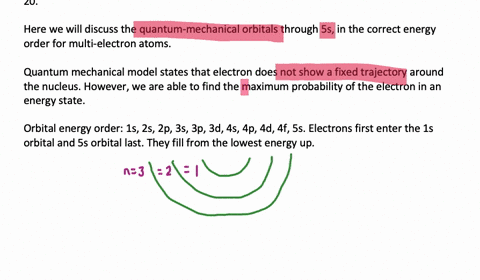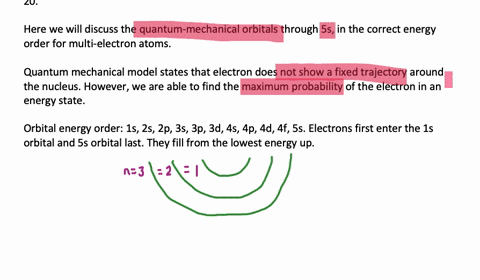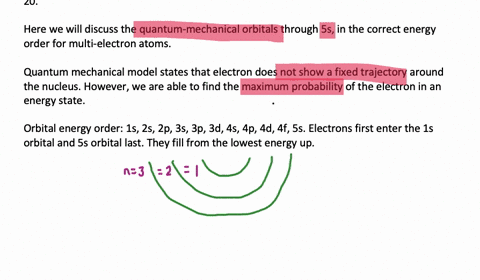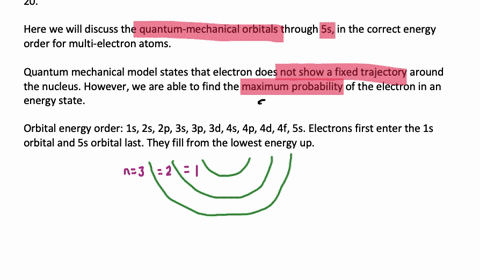
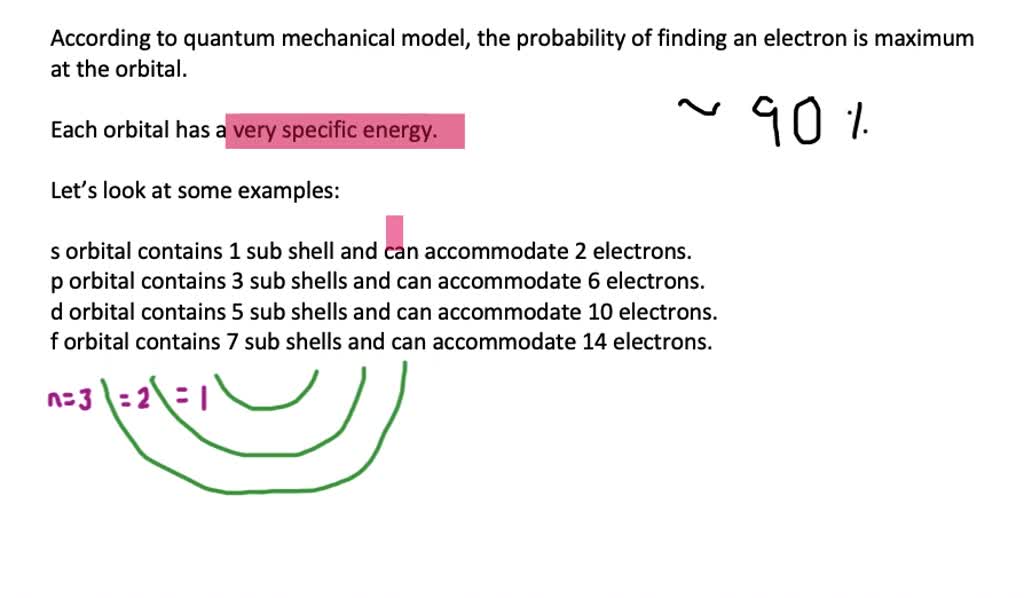
. So thats zero all the way up to n minus one. Answer The subshells are s 1 orbital which contains a maximum of 2 electrons. 1 3 5 7.
Electrons orbit the nucleus of an atom at different ranges called shells. Chemists describe the shell and subshell in which an orbital belongs with a two-character code such as 2p or 4fThe first character indicates the shell n 2. And f7 orbitals which.
Yes you Do you want list on a possible subsidy on a beetle associated with our principal quantum number and you qualify. The four possible subshells in the quantum mechanical models are s p d and f. List the four possible subshells in the quantum-mechanical model.
Learn more about Quantum Mechanical Model of Atom. See full answer below. The principal quantum number The principal quantum number n.
Should be zero as we do the river from them. List the four possible subshells in the quantum-mechanical model the number of orbitals in each subshell and the maximum number of electrons that can be contained in each subshell. Do page 128 in the Workbook.
List the four possible sub shells in the quantum-mechanical model the number of orbitals in each subshell and the maximum number of elections that can be contained in each subshell. Electron spin and the Stern-Gerlach experiment. P3 orbitals which contain a maximum of 6 electrons electrons.
Orbitals that have the same value of the principal quantum number form a shellOrbitals within a shell are divided into subshells that have the same value of the angular quantum number. This is the currently selected item. Essay answers are limited to about 500 words 3800 characters maximum including spaces.
Shells and Subshells of Orbitals. List all the possible subshells and orbitals associated with the principal quantum number n if n 4. Each energy level is given a number called the principal quantum number n.
Match the four structures with the four IR spectra. Each of these subshells is then broken up into orbitals. List the possible subshells for the n 6 shell.
The s p d and f subshells. These letters represent the numerical values of the angular momentum. An orbital is a wave function for an electron defined by the three quantum numbers n ℓ and m ℓ.
CH9 44 Sketch the shapes of the 3d orbitals. S P D F. So l is equal to zero l is equal to one l is equal to two and then n minus one thats four minus one thats equal to three.
P orbitals ℓ 1 are dumb-bell shaped. List two types of. So thats the last allowed value for l the angular momentum quantum number.
And once again if you have four here you get four allowed values. Give a possible set of values of the four quantum numbers for the 4s and 3d electrons in titanium. Learn more about Quantum Mechanical Model of Atom.
Electromagnetic spectrum is the range of all the possible frequencies of electromagnetic radiation. How do the 4d orbitals differ from the 3d orbitals. The three possible p orbitals are always perpendicular to each other.
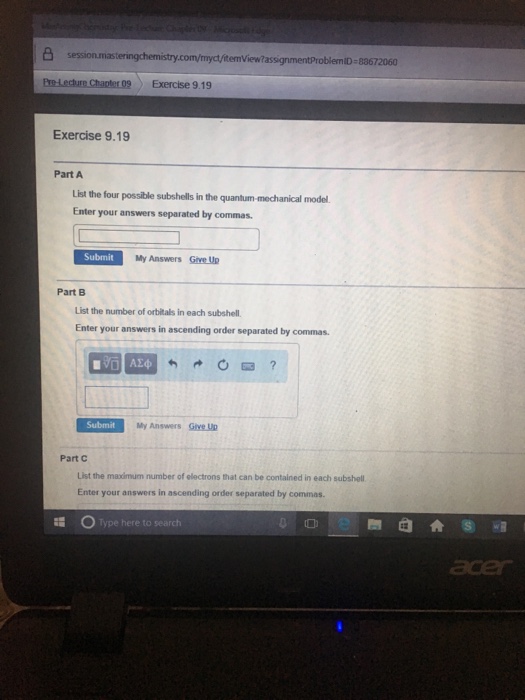
Chemistry questions and answers. 凸 commyctitemViewassignmentProblemiD-83672060 Pre-Lecture Chapter 09 Exercise 919 Exercise 919 Part A List the four possible subshells in the quantum-mechanical model Enter your answers separated by commas. View Untitled documentdocx from HUMN 1933 at Tulsa Community College.
Thinking about electrons as probabilistic matter waves using the de Broglie wavelength the Schrödinger equation and the Heisenberg uncertainty principle. CH9 19 For the four possible subshells in the quantum-mechanical model list the number of orbitals in each subshell and the maximum number of electrons that can be contained in each subshell. So this model is based on probability rather than certainty.
The allowed values are n 1 2 3 ldots. And if an equal five So were all here I should be zero one do 34 So Ill be though. Submit My Answers Give U Part B List the number of orbitals in each subshell.
Allowed values are l 0 1 2 n - 1 The angular momentum quantum number is related to the concept of subshells. The quantum mechanical model of the atom. For example the 3d subshell is in the n 3 shell the 2s.
Four numbers called quantum numbers were introduced to describe the characteristics of electrons and their orbitals. The fourth shell has four subshells. N Angular momentum quantum number.
Part A Explain why quantum-mechanical orbitals have fuzzy boundaries. 3785 Character s remaining none provided Exercise 919 Part A List the four possible subshells in the quantum-mechanical model. The four quantum numbers are the principle quantum number n the angular momentum quantum number l the magnetic quantum number ml and the electron spin quantum number ms.
The closest shell has a. The s subshell has 1. Angular momentum azimuthal quantum number.
The principal quantum number is related to the concept of shells. Orbitals define regions in space where you are likely to find electrons. The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons they are assigned four numbers called quantum numbers.
When were the Bohr model and the quantum-mechanical model for the atom developed. L Magnetic quantum number. No two electrons can be described by the exact same four quantum numbers.
S orbitals ℓ 0 are spherical shaped. Need a deep-dive on the concept behind this application. What are the allowed values for l.
The principle quantum number n describes the energy and distance from the nucleus and represents the shell. Each shell has a different energy level increasing the further it is from the nucleus. L This determines the shape of the probability distribution.
What purpose do these models serve. Introduction to the quantum mechanical model of the atom. We know l equals zero do and minus one.
Solved List The Four Possible Subshells In The Quantum Mechanical Model The Number Of Orbitals In Each Subshell And The Maximum Number Of Electrons That Can Be Contained In Each Subshell

Solved List The Four Possible Subshells In The Quantum Mechanical Model The Number Of Orbitals In Each Subshell And The Maximum Number Of Electrons That Can Be Contained In Each Subshell
Solved 凸 Com Myct Itemview Assignmentproblemid 83672060 Chegg Com
No comments for "List the Four Possible Subshells in the Quantum Mechanical Model"
Post a Comment